Identification of the glucosinolate transporter complement
Glucosinolates and Arabidopsis is an excellent model system to study the dynamic molecular interactions between components of the biosynthetic machinery distributed across several compartments, and to study how glucosinolates are translocated from production to storage site.
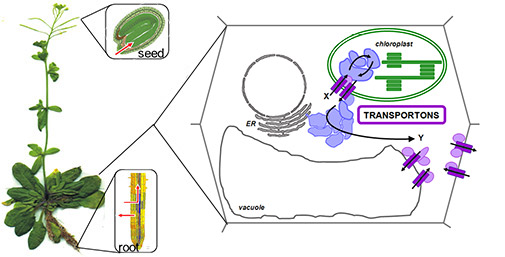
Intra- and intercellular transport of metabolites is essential for many processes, such as cellular homeostasis, cell communication with the surroundings and coordination of growth and defense responses in plants. Using the glucosinolate defense compounds in the model plant Arabidopsis thaliana, we aim to identify the first full transporter complement of given metabolites. This includes characterization of the multiple membrane barriers of the transport pathway – from when glucosinolates exit source tissues to they reach their destination.
Today, we have identified the plasmamembrane-localized glucosinolate importers, the GTRs, and exporters, UMAMITs, which are essential for intercellular distribution and seed accumulation. However, we still do not know how glucosinolates are glucosinolates are moved into the vacuole, exported out of epidermis, and whether they are remobilized to other tissues during development?
We will identify additional glucosinolate transporters by screening for transport activity using cDNA libraries expressed in oocytes of Xenopus laevis and by searching in transcriptomics databases. Candidate transporters will be validated in planta using loss-of-function mutants and grafting experiments with glucosinolate biosynthesis mutants and transporter mutants.
Ultimately, we expect to unravel how glucosinolates are translocated from production site to storage site, and to generate a dynamic model for movement of these compounds within and between organs in the plant throughout development.
Xu D, Hunziker P, Koroleva O, Blennow A, Crocoll C, Schulz A, Nour-Eldin HH, Halkier BA (2019 GTR-mediated radial import directs accumulation of defensive glucosinolates to sulfur-rich cells (S-cells) in phloem cap of inflorescence stem of Arabidopsis thaliana. Mol Plant. DOI: 10.1016/j.molp.2019.06.008
Hunziker P, Halkier BA, Schulz A (2019) Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development. J Exp Bot. DOI:10.1093/jxb/erz176
Larsen B, Xu D, Halkier BA, Nour-Eldin HH (2017) Advances in methods for identification and characterization of plant transporter function. J Exp Bot.68, 4045-4056. DOI: 10.1093/jxb/erx140
Nour-Eldin HH, Madsen SR, Engelen S, Jørgensen ME, Olsen CE, Andersen JS, Seynnaeve D, Verhoye T, Fulawka R, Denolf P, Halkier BA (2017) Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat Biotechnol. DOI: 10.1038/nbt.3823
Jørgensen ME, Nour-Eldin HH, Halkier BA (2015) Transport of defense compounds from source to sink: lessons learned from glucosinolates. Trends Plant Sci 20: 508–514. DOI: 10.1016/j.tplants.2015.04.006
Madsen SR, Olsen CE, Nour-Eldin HH, Halkier BA (2014) Elucidating the role of transport processes in leaf glucosinolate distribution. Plant Physiol 166: 1450–1462. DOI: 10.1104/pp.114.246249
Andersen TG, Nour-Eldin HH, Fuller VL, Olsen CE, Burow M, Halkier BA (2013) Integration of biosynthesis and long-distance transport establish organ-specific glucosinolate profiles in vegetative Arabidopsis. Plant Cell 25: 3133–3145. DOI: 10.1105/tpc.113.110890
Nour-Eldin HH, Andersen TG, Burow M, Madsen SR, Jørgensen ME, Olsen CE, Dreyer I, Hedrich R, Geiger D, Halkier BA (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488: 531–534. DOI: 10.1093/gbe/evs063
Video
Video abstract of Transport of defense compounds from source to sink: lessons learned from glucosinolates.
Contact Persons
 |
Professor |
 |
Scientific consultant |
